바이오하모니 의약품 데이터 스토어 FAQ
1
BioHarmony는 보고서에 대한 데이터를 어디에서/어떻게 얻습니까?
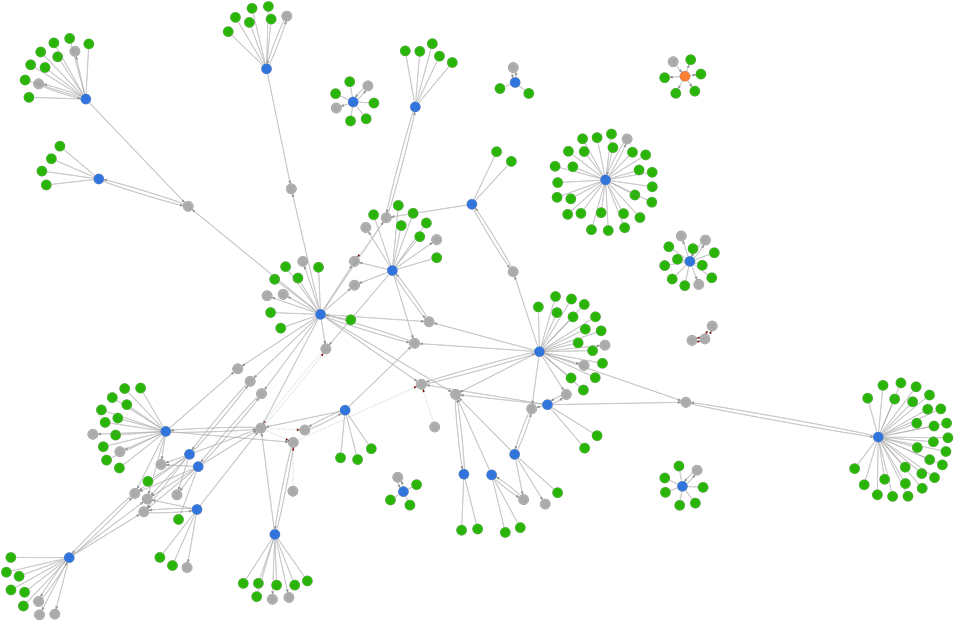
바이오하모니의 의미 론 네트워크 그래프의 예입니다. 파란색 및 주황색 원은 데이터 원본을 나타냅니다. 녹색 원은 각 소스에서 추출한 정보를 나타냅니다. 회색 원은 여러 데이터 원본에 걸쳐 정보를 연결하는 데 사용할 수 있는 모호하지 않은 의미 체계 식별자를 나타냅니다.
2
바이오하모니가 다른 약물 데이터베이스와 다른 이유는 무엇입니까?
- 검색 파이프라인의 모든 단계에서 조화를 이수합니다. 전임상, 임상 또는 사후 승인 등 파이프라인에서의 위치에 관계없이 BioHarmony는 모델과 의사 결정을 개선할 정보를 캡처합니다. BioHarmony는 다음과 같은 정보를 포함시켜 신뢰할 수 있고 전체적인 보기를 제공합니다.
-
- 클레임 상환: BioHarmony는 약물 분류 및 적응증에 대한 세부 정보를 제공하여 해부학 치료 화학 (ATC), 국제 질병 분류 - 10 개정 (ICD-10), 의료 공통 절차 코딩 시스템 (HCPCS)을 포함한 확립 된 클레임 코드에 각각 연결합니다.
-
- 의약품 수익 보고서: BioHarmony는 주요 의약품 제조업체의 연간 및 분기별 수익 보고서를 제공합니다.
- 실시간 세부 정보. 알고리즘은 정확하고 최신 의 추세를 보장하기 위해 매일 실행됩니다.
- 정확하고 선별된 데이터. BioHarmony의 데이터는 정확성을 보장하기 위해 생물학 및 화학 전문가에 의해 주기적으로 검사됩니다.
- 기계를 읽을 수 있습니다. 모델링 및 정보학에 사용하도록 설계된 당사의 데이터는 연결된 의미 체계 식별자가 포함된 기계 판독 가능한 형식으로 제공됩니다.
- 캡처된 메타데이터. BioHamony의 독자적인 기술은 구조화 된 데이터베이스와 비정형 데이터 원본을 모두 통과합니다. 이러한 비정형 리소스(메타데이터)의 귀중한 데이터를 기계 판독 모델에 통합할 수 있는 기계 판독 가능한 형식으로 자동으로 변환합니다.
3
바이오하모니 데이터베이스에 얼마나 많은 약물이 있습니까?
현재 BioHarmony에는 모든 FDA 및 EMA 승인 약물에 대한 보고서와 임상 시험에서 수천 개의 약물이 포함되어 있습니다! 당사에 연락하여 추가 데이터 추가를 요청할 수 있습니다.
4
대량 할인을 받을 수 있나요?
BioHarmony는 사용자 계정당 모든 약물 보고서에 매월 액세스할 수 있는 편리한 온라인 구독을 제공합니다. 많은 수의 사용자 계정을 확보하는 데 관심이 있는 경우sales@biometadata.com 도움을 받으십시오..
5
BioHarmony에서 수집한 공개 데이터와 독점 데이터를 통합할 수 있습니까?
예! 안전한 엔터프라이즈 솔루션과 특정 요구 사항을 논의하기 위해 info@biometadata.com 문의하십시오.
6
개별 BioHarmony 약물 보고서에 추가 정보를 추가할 수 있습니까?
7
BioHarmony 데이터베이스를 통해 특정 약물 표적 또는 질병 정보를 검색할 수 있습니까?
절대로! BioHarmony 응용 프로그램의 검색 기능은 활성 성분, 질병, 약물 클래스, 회사, 단백질 이름 및 유전자 기호, MeSH 식별자, ICD-10 코드, ATC 코드 등을 포함한 다양한 유형의 키워드를 지원합니다. BioHarmony는 특정 검색어와 관련된 사용 가능한 보고서를 나타냅니다.
8
바이오하모니의 사용 사례는 무엇입니까?
BioHarmony는 약물의 수명 주기 전반에 걸쳐 다양한 단계에서 워크플로우를 지원할 수 있습니다. 보고서에는 연구 팀, 비즈니스 전문가 및 안전 업무와 관련된 데이터가 포함되어 있습니다. 여기에서 몇 가지 사례 연구를 읽을 수 있습니다.
9
학문으로서 구독 할인을 받을 수 있나요?
과학 연구를 지원하는 일환으로, 우리는 학술 이메일로 무료 1 년 표준 구독을 제공하고 있습니다.
청구하려면 체크 아웃 페이지에서 "쿠폰 을 받으세요?" 섹션에 "아카데미"를 입력합니다.
.png?width=250&height=56&name=BioHarmony-White-Logo%20(2).png)