Hyaluronidase
Amphadase, Darzalex Faspro, Herceptin Hylecta, Phesgo, Vitrase (hyaluronidase) is an enzyme pharmaceutical. Hyaluronidase was first approved as Vitrase on 2004-05-05. It is used to treat extravasation of diagnostic and therapeutic materials in the USA.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
Amphadase, Vitrase (discontinued: Hydase)
CombinationsDarzalex faspro, Herceptin hylecta, Phesgo
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Daratumumab
+
Hyaluronidase
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Darzalex Faspro | daratumumab and hyaluronidase-fihj | Johnson & Johnson | N-761145 RX | 2020-05-01 | 1 products |
Hyaluronidase
Hyaluronidase
+
Pertuzumab
+
Trastuzumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Phesgo | pertuzumab, trastuzumab, and hyaluronidase-zzxf | Genentech | N-761170 RX | 2020-06-29 | 2 products |
Hyaluronidase
+
Trastuzumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Herceptin Hylecta | trastuzumab and hyaluronidase-oysk | Genentech | N-761106 RX | 2019-02-28 | 1 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| amphadase | Biologic Licensing Application | 2020-05-28 |
| darzalex faspro | Biologic Licensing Application | 2022-01-26 |
| herceptin hylecta | Biologic Licensing Application | 2020-10-19 |
| phesgo | Biologic Licensing Application | 2020-06-29 |
| vitrase | Biologic Licensing Application | 2018-05-29 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| extravasation of diagnostic and therapeutic materials | — | D005119 | — |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
daratumumab / hyaluronidase, Darzalex Faspro, Janssen Biotech, Inc. | |||
| 2028-01-15 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J1575 | Injection, immune globulin/hyaluronidase, (hyqvia), 100 mg immuneglobulin |
| J3470 | Injection, hyaluronidase, up to 150 units |
| J3471 | Injection, hyaluronidase, ovine, preservative free, per 1 usp unit (up to 999 usp units) |
| J3472 | Injection, hyaluronidase, ovine, preservative free, per 1000 usp units |
| J3473 | Injection, hyaluronidase, recombinant, 1 usp unit |
| J9144 | Injection, daratumumab, 10 mg and hyaluronidase-fihj |
| J9311 | Injection, rituximab 10 mg and hyaluronidase |
| J9316 | Injection, pertuzumab, trastuzumab, and hyaluronidase-zzxf, per 10 mg |
| J9356 | Injection, trastuzumab, 10 mg and hyaluronidase-oysk |
Clinical
Clinical Trials
129 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | D012559 | EFO_0000692 | F20 | — | 1 | 9 | 15 | 8 | 33 |
| Delirium | D003693 | R41.0 | — | 7 | 12 | 7 | 4 | 27 | |
| Psychotic disorders | D011618 | F20.81 | 1 | 1 | 4 | 7 | 1 | 14 | |
| Psychomotor agitation | D011595 | — | — | 1 | 2 | 2 | 5 | ||
| Bipolar disorder | D001714 | EFO_0000289 | F30.9 | — | — | 2 | 1 | 1 | 4 |
| Nausea | D009325 | HP_0002018 | R11.0 | 1 | — | 1 | 2 | — | 4 |
| Postoperative nausea and vomiting | D020250 | EFO_0004888 | — | — | — | 2 | 1 | 3 | |
| Migraine disorders | D008881 | EFO_0003821 | G43 | — | — | — | 3 | — | 3 |
| Cognitive dysfunction | D060825 | G31.84 | — | — | 1 | 1 | 1 | 3 | |
| Depression | D003863 | F33.9 | — | — | 1 | 1 | — | 2 |
Show 15 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Neoplasms | D009369 | C80 | — | 3 | 4 | — | 1 | 6 | |
| Confusion | D003221 | F44.89 | — | — | 2 | — | — | 2 | |
| Critical illness | D016638 | — | 1 | 1 | — | — | 2 | ||
| Dementia | D003704 | F03 | — | — | 1 | — | — | 1 | |
| Psychophysiologic disorders | D011602 | F45.9 | — | — | 1 | — | — | 1 | |
| Anxiety disorders | D001008 | EFO_0006788 | F41.1 | — | — | 1 | — | — | 1 |
| Mood disorders | D019964 | EFO_0004247 | F30-F39 | — | 1 | 1 | — | — | 1 |
| Medication adherence | D055118 | EFO_0006344 | — | — | 1 | — | — | 1 | |
| Hip fractures | D006620 | EFO_0003964 | S72.00 | — | — | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Movement disorders | D009069 | EFO_0004280 | G25 | — | 1 | — | — | 1 | 2 |
| Intellectual disability | D008607 | F73 | — | 1 | — | — | — | 1 | |
| Artificial respiration | D012121 | — | 1 | — | — | — | 1 | ||
| Critical care | D003422 | — | 1 | — | — | — | 1 | ||
| Ventilator weaning | D015300 | — | 1 | — | — | — | 1 | ||
| Gambling | D005715 | F63.0 | — | 1 | — | — | — | 1 | |
| Drug-induced akathisia | D017109 | EFO_1000903 | G25.71 | — | 1 | — | — | — | 1 |
| Cognition disorders | D003072 | — | 1 | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Dyslipidemias | D050171 | HP_0003119 | — | — | — | — | 2 | 2 | |
| Insulin resistance | D007333 | EFO_0002614 | — | — | — | — | 2 | 2 | |
| Stroke | D020521 | EFO_0000712 | I63.9 | — | — | — | — | 1 | 1 |
| Female genital diseases | D005831 | EFO_0009549 | N85 | — | — | — | — | 1 | 1 |
| Recurrence | D012008 | — | — | — | — | 1 | 1 | ||
| Obesity | D009765 | EFO_0001073 | E66.9 | — | — | — | — | 1 | 1 |
| Metabolic syndrome | D024821 | EFO_0000195 | E88.81 | — | — | — | — | 1 | 1 |
| Lactic acidosis | D000140 | EFO_1000036 | E87.20 | — | — | — | — | 1 | 1 |
| Patient compliance | D010349 | — | — | — | — | 1 | 1 | ||
| Chorea | D002819 | EFO_0004152 | G25.5 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | HYALURONIDASE |
| INN | — |
| Description | Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid. Karl Meyer classified these enzymes in 1971, into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyaluronidases are two classes of eukaryotic endoglycosidase hydrolases and a prokaryotic lyase-type of glycosidase.
|
| Classification | Enzyme |
| Drug class | enzymes |
| Image (chem structure or protein) | 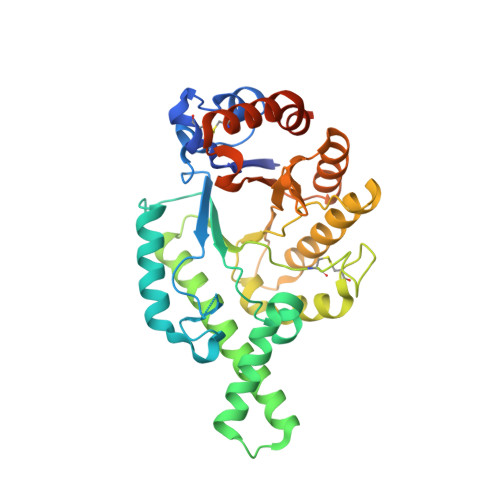 |
| Structure (InChI/SMILES or Protein Sequence) | — |
Identifiers
| PDB | 1FCQ, 1FCU, 1FCV, 1I8Q, 1LOH, 1LXK, 1LXM, 1N7N, 1N7O, 1N7P, 1N7Q, 1N7R, 1OJM, 1OJN, 1OJO, 1OJP, 1W3Y, 2ATM, 2BRP, 2BRV, 2BRW, 2C3F, 2CBI, 2CBJ, 2DP5, 2J1A, 2J1E, 2J62, 2J7M, 2J88, 2JNK, 2OZN, 2PE4, 2WB3, 2WDB, 2WH7, 2YVV, 2YW0, 2YX2, 3EKA, 4D0Q, 4UFQ, 5DIY, 6WV2, 6WWX, 6WXA, 6X3M, 7EYO, 8C6I |
| CAS-ID | — |
| RxCUI | — |
| ChEMBL ID | CHEMBL1201636 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00070 |
| UNII ID | — |
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
Phesgo - Roche
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 29,311 documents
View more details
Safety
Black-box Warning
Black-box warning for: Herceptin hylecta, Phesgo
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
21,466 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
