Simulect(basiliximab)
Simulect (basiliximab) is an antibody pharmaceutical. Basiliximab was first approved as Simulect on 1998-05-12. It has been approved in Europe to treat graft rejection and kidney transplantation. It is known to target interleukin-2 receptor subunit alpha.
Download report
Favorite
Novartis Pharmaceuticals
Commercial
Trade Name
FDA
EMA
Simulect
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Basiliximab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Simulect | basiliximab | Novartis Pharmaceuticals Corporation | N-103764 RX | 1998-05-12 | 2 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| simulect | Biologic Licensing Application | 2021-02-12 |
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
basiliximab, Simulect, Novartis Pharmaceuticals Corporation | |||
| 2105-05-12 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J0480 | Injection, basiliximab, 20 mg |
Clinical
Clinical Trials
94 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Kidney transplantation | D016030 | 1 | 9 | 8 | 9 | 2 | 26 | ||
| Liver transplantation | D016031 | EFO_0010682 | — | 1 | 5 | 3 | — | 9 | |
| Beta-thalassemia | D017086 | Orphanet_848 | D56.1 | — | — | — | 3 | — | 3 |
| Chronic kidney failure | D007676 | EFO_0003884 | N18.6 | — | 2 | 1 | 1 | — | 3 |
| Chronic renal insufficiency | D051436 | N18 | — | 1 | — | 1 | — | 2 | |
| Cytomegalovirus infections | D003586 | EFO_0001062 | B25 | — | — | — | 1 | — | 1 |
| Glomerulonephritis | D005921 | N05 | — | — | — | 1 | — | 1 | |
| Diabetes mellitus | D003920 | EFO_0000400 | E08-E13 | — | — | — | 1 | — | 1 |
| Liver cirrhosis | D008103 | EFO_0001422 | K74.0 | — | — | — | 1 | — | 1 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes mellitus | D003922 | EFO_0001359 | E10 | — | 6 | 2 | — | 1 | 9 |
| Reperfusion injury | D015427 | — | — | 1 | — | — | 1 | ||
| Graft rejection | D006084 | — | — | 1 | — | — | 1 | ||
| Chronic obstructive pulmonary disease | D029424 | EFO_0000341 | J44.9 | — | — | 1 | — | — | 1 |
| Emphysema | D004646 | EFO_0000464 | J43 | — | — | 1 | — | — | 1 |
| Alpha 1-antitrypsin deficiency | D019896 | E88.01 | — | — | 1 | — | — | 1 | |
| Infections | D007239 | EFO_0000544 | — | — | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Myeloid leukemia acute | D015470 | C92.0 | 2 | 1 | — | — | 1 | 4 | |
| Hodgkin disease | D006689 | C81 | 1 | 2 | — | — | 1 | 4 | |
| Non-hodgkin lymphoma | D008228 | C85.9 | 1 | 1 | — | — | 1 | 3 | |
| Precursor cell lymphoblastic leukemia-lymphoma | D054198 | C91.0 | 1 | 1 | — | — | 1 | 3 | |
| Graft vs host disease | D006086 | D89.81 | — | 1 | — | — | 1 | 2 | |
| Ulcerative colitis | D003093 | EFO_0000729 | K51 | — | 2 | — | — | — | 2 |
| Primary myelofibrosis | D055728 | D47.4 | — | 1 | — | — | 1 | 2 | |
| Multiple myeloma | D009101 | C90.0 | — | 1 | — | — | 1 | 2 | |
| B-cell chronic lymphocytic leukemia | D015451 | C91.1 | — | 1 | — | — | 1 | 2 | |
| Bcr-abl positive chronic myelogenous leukemia | D015464 | EFO_0000340 | — | 1 | — | — | 1 | 2 |
Show 14 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Brain neoplasms | D001932 | EFO_0003833 | C71 | 1 | — | — | — | — | 1 |
| Myelodysplastic syndromes | D009190 | D46 | 1 | — | — | — | — | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | BASILIXIMAB |
| INN | basiliximab |
| Description | Basiliximab (chimeric mab) |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 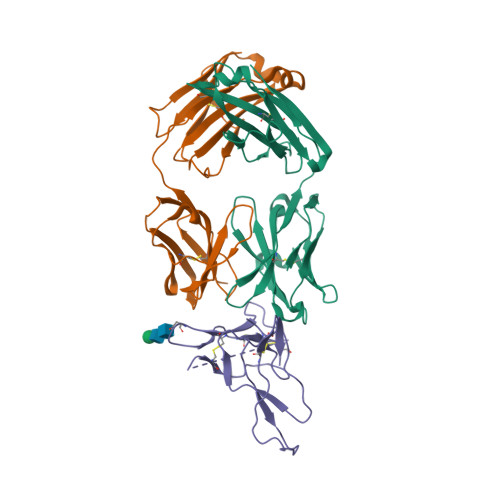 |
| Structure (InChI/SMILES or Protein Sequence) | >3IU3:A,C,H|Heavy chain of Fab fragment of Basiliximab
QLQQSGTVLARPGASVKMSCKASGYSFTRYWMHWIKQRPGQGLEWIGAIYPGNSDTSYNQKFEGKAKLTAVTSASTAYME
LSSLTHEDSAVYYCSRDYGYYFDFWGQGTTLTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGA
LTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKRVEP
>3IU3:B,D,L|Light chain of Fab fragment of Basiliximab
QIVSTQSPAIMSASPGEKVTMTCSASSSRSYMQWYQQKPGTSPKRWIYDTSKLASGVPARFSGSGSGTSYSLTISSMEAE
DAATYYCHQRSSYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESV
TEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGE |
Identifiers
| PDB | 3IU3 |
| CAS-ID | 179045-86-4 |
| RxCUI | 196102 |
| ChEMBL ID | CHEMBL1201439 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00074 |
| UNII ID | 9927MT646M (ChemIDplus, GSRS) |
Target
Agency Approved
No data
Alternate
IL2RA
IL2RA
Organism
Homo sapiens
Gene name
IL2RA
Gene synonyms
NCBI Gene ID
Protein name
interleukin-2 receptor subunit alpha
Protein synonyms
CD25, IL-2 receptor subunit alpha, IL-2R subunit alpha, interleukin 2 receptor, alpha, p55, TAC antigen
Uniprot ID
Mouse ortholog
Il2ra (16184)
interleukin-2 receptor subunit alpha (Q61731)
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 5,389 documents
View more details
Safety
Black-box Warning
Black-box warning for: Simulect
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
3,020 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
